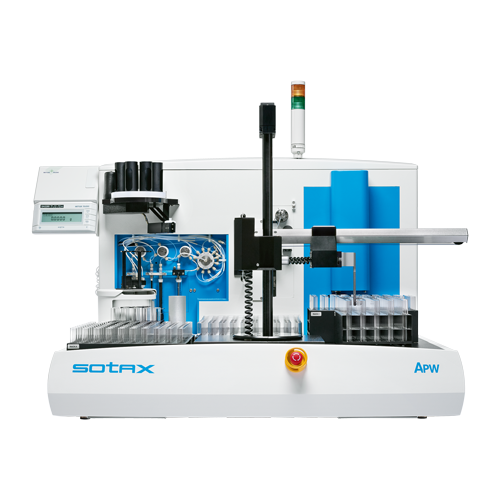
About Us
NIKYANG Founded in 1998, NIKYANG Enterprise Limited has developed a reputation for dynamic leadership, satisfying our customers with innovative one-stop fully-automated laboratory solutions and support. Our state-of-the-art products and services make a major contribution to promoting lab efficiency, modernization, and consistency of results across a range of industries in Asia. Headquartered in Hong Kong, we support our customers through an